Univ. Prof. Dipl. Ing. Dr. techn. Marko D. Mihovilovic
Vienna University of Technology
Project I - Development of a Platform of Photoswitchable Monoamine Transporter Ligands
Project I - Development of a Platform of Photoswitchable Monoamine Transporter Ligands
During the current funding period, photo-switchable inhibitors for monoamine transporters were successfully designed, synthesized, characterized and biologically studied (in close cooperation with the Sitte lab) for the first time. The currently available designs for SERT shall be extended towards compounds selectively acting on DAT and NET (and eventually also on GAT1). Further refinement of the designs will be based on theoretical studies (in cooperation with the Ecker and González labs).
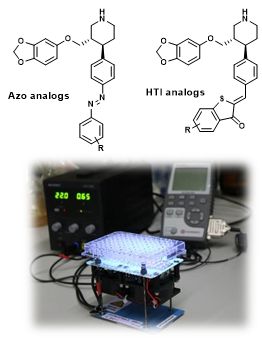
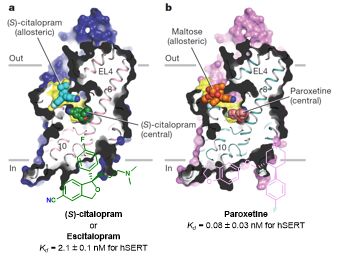
Based on the structures of SERT with bound ligands escitalopram and paroxetine obtained by x-ray diffraction, computational models were developed to identify suitable attachment positions for photo¬switchable substituents (such as azobenzene or hemithioindigo). The predicted analogs with sufficient space within the binding site were synthesized and characterized regarding their photoswitching properties (half-lives, absorption wavelength, etc.). For the biological assessment, a screening device employing LED-technology to trigger the switch was developed in-house. The data acquisition regime was optimized to ensure reproducible determination of the functional photoswitching properties. This work-flow will be extended towards efficient ligands for DAT and NET (eventually also GAT1) from the literature in order to establish a suite of selective and photoswitchable ligands for mechanistic studies of the transporter system.
Workplan: In this project, computational analysis based on homology models of the transporters will be developed. After the identification of suitable substitution sites to introduce the photoswitch motif, retrosynthetic analysis will be conducted in order to outline a plan for the forward synthesis. Total synthesis of the switchable analog will take advantage of lab-automation in the Mihovilovic group. A protocol for the facile determination of key parameters of the photoswitch is already in place from the current funding period and can be conducted in a parallelized format. Feed-back from the theoretical groups will be implemented in order to adjust in particular half-lives and switching wavelengths to the particular requirements. The pharmacological characterization will be carried out in the Sitte lab.
Networking: The project is a joint effort of the Mihovilovic, Sitte, González, and Ecker labs. Computational studies on the receptor will be conducted in the Ecker group and will be complemented by theoretical expertise for molecular entities from the González lab. All compound synthesis and characterization will be carried out in the Mihovilovic group. Compound testing and pharmacological characterization of switchable compounds will be performed at the Sitte lab. The methodology developed within this work package can also be transferred to the preparation of conceptually related compounds for the Danzl group to contribute in the solution of pending research questions, there, representing a side-line project for the PhD candidate.
Methods: The student will learn the full repertoire of state-of-the-art synthetic organic chemistry and analytical methods including NMR, HPLC, GC-MS, LC-MS-NMR. Furthermore the student will be trained in the application of the house-built irradiation device and determination of biological data. Photoswitching studies will largely exploit UV/Vis spectroscopy.
Project II - Photochemically Susceptible PIP-Analogs for the Investigation of Peptide Assemblies
Project II - Photochemically Susceptible PIP-Analogs for the Investigation of Peptide Assemblies
Assembly of proteins to supramolecular complexes is crucial for the formation of signalling complexes, by generating modules with new functionality. In cell membranes, such protein assemblies are often connected not via the polypeptide chains, but by lipids. The mechanisms, by which lipids influence membrane protein interactions, however, are controversially discussed: direct binding, local phase separation, the presence of boundary lipids, or the induction of local curvature are typically considered when explaining the forces that keep proteins in an assembly. In this project, we aim at determining how phosphoinositides (PIs) influence the cohesion of protomers in homo-oligomers of membrane transporters. The project is based on previous findings that binding of PIs alters the functionality of the serotonin transporter (SERT) and influences the formation of oligomeric complexes. A long-term aim of this project is to pinpoint, which PIs are able to interconnect SERT protomers or affect their function, and at which subcellular location the binding occurs.
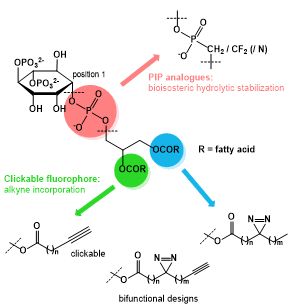
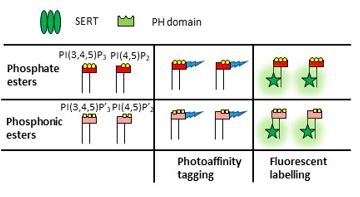
Workplan: In this project, the synthesis of the required tool compounds will be conducted. In order to increase stability of PIP2 complexes, hydrolytic degradation will be decreased by converting phosphate esters (esp. the bridging P to the lipid section, but in analogous fashion also the other phosphate functions) to phosphonic ester analogs. This approach offers the advantage, that PIP2-analogs can be administered in varying concentrations to facilitate aggregation behavior of the target biomolecules at the membranes. Most suitable candidates will be considered for further chemical modification towards installation of a photoaffinity. This strategy has been established for receptor-capture approaches in target identification; however, it also seems highly appropriate to further corroborate the complex stabilizing activity of PIs, which has been applied in context of lipid binding proteins recently. Upon irradiation, the diaziridin structural motif forms highly reactive radicals to covalently bind to amino-acids of the receptor binding site, converting a reversible binding process to a stable covalent system. Based on this rationale, establishing a covalently linked complex using a synthetic PI-analog should facilitate the long-term study of the formed complexes independent of endogenous PIP2.
In a prospective extension, fluorescent dyes of various absorption frequencies can be incorporated by the [3+2] cycloaddition of an azide with an alkyne upon Cu-catalysis (“click-chemistry”), further modifying the stearic acid by a carboxylic ester bearing a terminal alkyne group.
Networking: The project is a joint effort of the Mihovilovic and Sitte labs and will be complemented by Prof. G.J. Schütz as external partner. All synthesis and compound characterization will be conducted in the Mihovilovic group. Functional readout of the in vitro systems will be determined by the Sitte group. The external partner Schütz group will contribute by live cell single molecule fluorescence microscopy. We will synthesize novel types of PI analogs that are i) metabolically inert, ii) contain photo crosslinkers, and/or iii) are fluorescently labelled (Marko D. Mihovilovic). Using in vitro biochemistry, functional readout (Harald H. Sitte) and live cell single molecule fluorescence microscopy (Gerhard J. Schütz). The methodology developed within this work package can also be transferred to the preparation of conceptually related compounds for the Novarino group to contribute in the solution of pending research questions, there, representing a side-line project for the PhD candidate.
Methods: The student will learn the full repertoire of state-of-the-art synthetic organic chemistry and analytical methods including NMR, HPLC, GC-MS, LC-MS-NMR. Furthermore the student will be exposed to photoaffinity labelling techniques and will also determine preliminary biological data.
Project III - Developing a Tool Compound Platform of Labelled Ligands for Mechanistic Studies on the GABAA-Receptor
During the current funding period, several novel chemo-types for GABAA-receptor modulators were identified and characterized, enabling access to a number of tool compounds. This platform of structures (in combination with data from the literature) provides a unique opportunity for intensifying mechanistic studies in the area and obtaining a more comprehensive picture of binding sites and properties. However, for more in-dept investigations additional information on the binding mode of these compounds is required. Based on novel chemotypes identified in particular during funding period 2 (in particular within the ring-opened indole bioisoster scaffold), photo-switchable GABAA-modulators seem within reach. This provides a unique opportunity to exploit the concept of photopharmacology towards mechanistic studies at this complex system. Moreover, the current re-organization of the radio-chemistry lab at the nuclear reactor site (Atominstitut) of VUT in Prater provides a unique opportunity to also include studies involving a large diversity of radio nuclei in context of GABAA receptor pharmacology.
Workplan: In this project, focused compound library design is envisioned in order to further improve novel chemotypes of the previous funding period. A major focus along this line is put on the exploitation of the newly set-up synthesis robot within the group for focussed compound library design. This will significantly boost the output of compounds for SAR studies and will be substantially complemented by computational analysis based on homology models available within the consortium, enabling identification of prospective substitution sites to introduce photoswitches. Additionally, synthetic activities will be extended towards providing radio-labelled tool compounds for pharmacological studies based on the available facilities at the nuclear reactor site of VUT.
Networking: The project is a joint effort of the Mihovilovic (synthesis), Ernst (pharmacology), and Ecker as well as González labs (computational chemistry), extending fruitful collaborations of both previous funding periods. Additionally, on-site expertise at VUT in the field of automation supported synthesis will be complemented together with unique know-how in radio-chemistry.
Methods: The student will learn the full repertoire of state-of-the-art synthetic organic chemistry and analytical methods including NMR, HPLC, GC-MS, LC-MS-NMR. A major focus rests on the application of the newly set-up synthesis robot in the group for fast-track access to focussed compound libraries. There are strong synergies with the projects of the other MolTag-PhD students within the Mihovilovic group. Access to the radio-chemistry unit at the research reactor of VUT offers a USP for this project, in particular combined with the strong expertise of the group in continuous flow-chemistry: Due to close connection to the research reactor all regulatory requirements for dealing with high activities and practically all radionuclides are fulfilled. Considering that many research reactors and the associated radiochemical laboratories in western and central Europe were closed in the last decade, the close connection of the radiochemical facility to a reactor for isotope production is rather unique (not only in Austria). Overall, this enables the production and/or use of many radionuclides that are not commonly available, for various diagnostic and therapeutic applications. These include (but are not limited to) zirconium-89, ruthenium-103, copper-64, manganese-52 and cobalt-55 for PET imaging (e.g. ImmunoPET, late time-point imaging of peptides, affibodies, antibodies), and therapeutic radionuclides such as actinium-225, astatine-211, luthethium-177, tin-117, yttrium-90 or iodine-131.
