The study is first authored by MolTag alumna Konstantina Bampali from the Ernst lab at MedUni Wien and involves among others the current MolTag PhD student Florian Vogel.
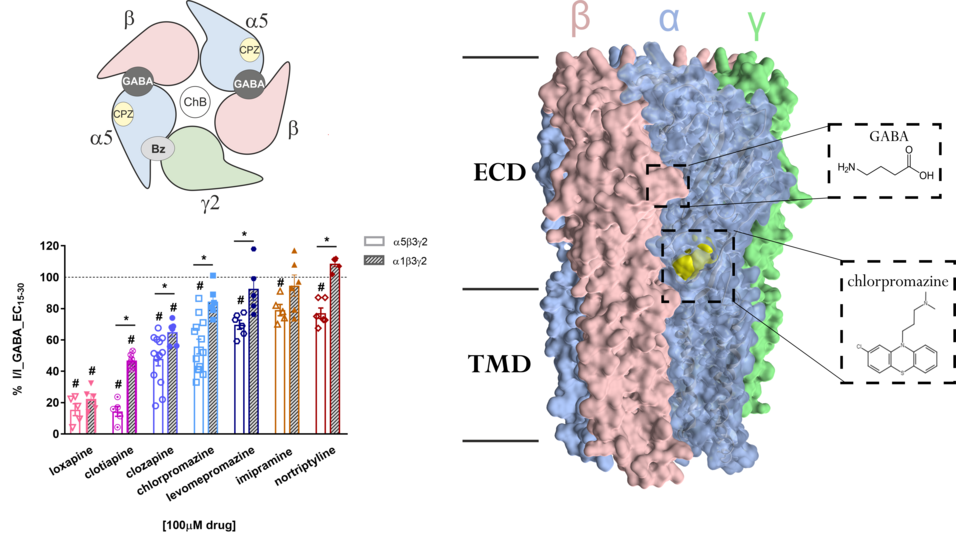
Clozapine and chlorpromazine, and other tricyclic antipsychotic and antidepressant molecules, inhibit currents in GABAA receptors. Our study shows for the first time that these drugs inhibit GABA-elicited currents in a hippocampal, α5-containing subtype, which might contribute to clinically observed drug effects. Intriguingly, the mechanisms are different - chlorpromazine acts at a novel allosteric site in α5 GABAA receptor subunits, while clozapine acts as an atypical orthosteric antagonist.
Konstantina Bampali, Filip Koniuszewski, Luca Silva, Sabah Rehman, Florian Vogel et al. Tricyclic antipsychotics and antidepressants can inhibit α5-containing GABAA receptors by two distinct mechanisms.
accepted in British Journal of Pharmacology
Authorea. January 12, 2022. DOI: 10.22541/au.161797799.93015888/v2
Klick on the picture for getting directly to the study!
Congratulations to Konstantina and the team!