Stefanie Kickinger is a member of the MolTag Doctoral Program since 2016, supervised by Gerhard Ecker, Pharminfo Group at University of Vienna.
She did her internship abroad for 5 months in the lab of William L Jorgensen; Dept. of Chemistry, Yale University; and for one month in the lab of Petrine Wellendorph, Dept. of Drug Design and Pharmacology in Kopenhagen.
Stefanies lab rotation was 2016 in the lab of her co-supervisor, Margot Ernst, at Medical University.
In 2017/18, Steffi was a student representative and co-organised MolTag Workshop for students.
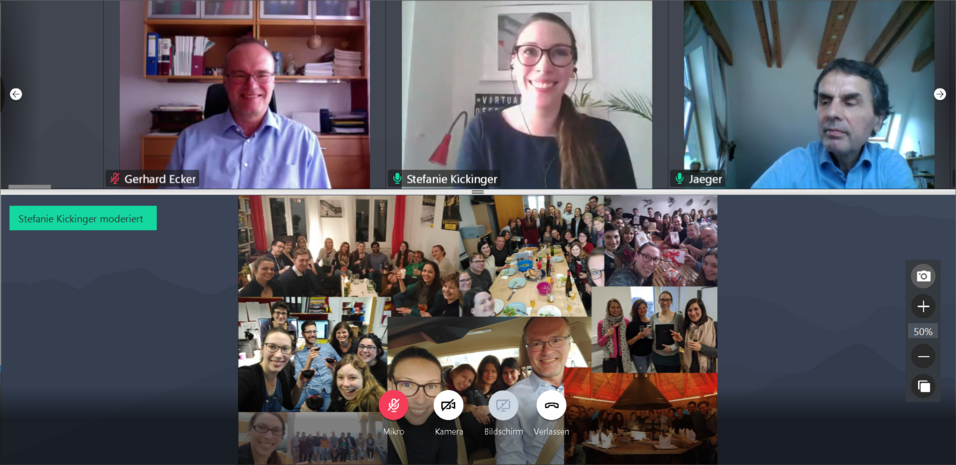
Due to the strange times we currently live in, Stefanie had to do a remote defense. Her presentation was very well attended with round 80 listeners: examiners, colleagues, friends and family from near and far. Everyone also celebrated with Stefanie from home to celebrate her great success
So, let's raise a glass and hip-hip-hooray for another great defense in the MolTag community!
Congratulations, Dr.in Stefanie Kickinger!